43rd ECFS Cystic Fibrosis Online European Conference
The scientific developments in the disease were significant,
according to the 43rd ECFS Cystic Fibrosis Pan-European Congress, which was attended by representatives of the Hellenic Cystic Fibrosis Association and attended by over 3,700 participants from more than 72 countries with health scientists, researchers, academics, company representatives and patient representatives from all over Europe. This year’s conference again covered a variety of topics and was aimed at health scientists and experts as well as patients.
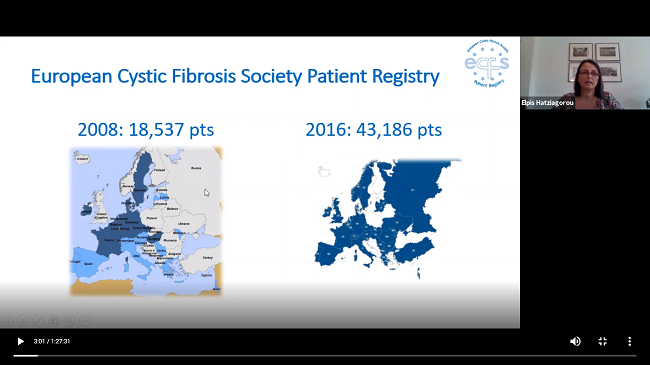
In the first instance, special mention was made of the progress made by the European Cystic Fibrosis Patient Registry, which registered 50,822 patients from 38 countries in Europe for the year 2018 with an additional 6,000 patients and 7 countries more than in 2017.
The impact of the coronavirus pandemic has not left C.F. patients in Europe unaffected. During the first wave of the pandemic 162 patients were recorded as having coronavirus, of which 115 recovered and 3 died, and 93 cases were mild in contrast to 5 that were critically ill. In addition, it was observed that while coronavirus cases in the general population in Europe have been increasing since 4 May, mortality has decreased significantly. The scientists’ conclusions were very reassuring, according to which the incidence of coronavirus cases in the Cystic Fibrosis community in Europe is not as high as expected, and the coronavirus does not have as great an effect on patients in the end.
Also many European CVI centres mentioned the importance of using digital technologies to monitor patients during the difficult period of the pandemic, thanks to which patients using special devices (such as individual spirometers, oximeters, etc.) can digitally send all medical data about their clinical condition to their doctors without interrupting their regular monitoring due to the pandemic.
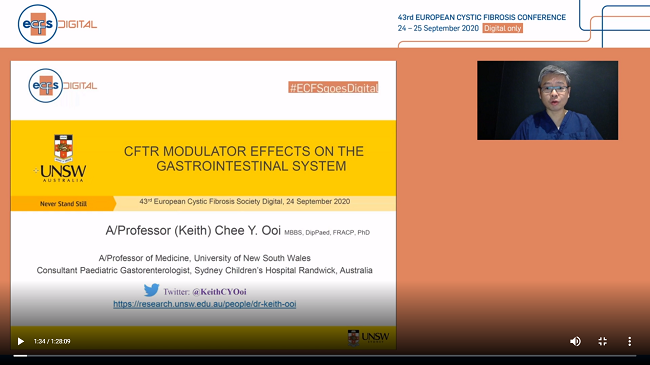
The new data of clinical researches on the effect of the new combinations of CFTR modifier drugs (Orkambi, Symkevi, Kalydeco, Kaftrio) targeting the causes of the disease and affecting the majority of mutations in patients with CF were also presented at the conference. As it was observed, these drugs have beneficial effects on other systems of the body of the patients receiving them, besides the respiratory system, such as the gastrointestinal system.
In particular, after the administration of Kaftrio, a change in the gut microbiota and an improvement in digestion is observed, resulting in an increase in body mass index (BMI), as well as a possible reduction or increase in the incidence of pancreatitis, depending on the patient’s condition before administration.
Particular reference was also made to patients receiving Orkambi, with epidemiological data to date showing that patients with FEV1 below 40% do not show a significant change in pulmonary exacerbations, while the rest show a significant reduction in exacerbations and hospitalizations.
An effect has also been observed on the reproductive system, with a characteristic increase in the number of women with Cystic Fibrosis receiving the new treatments and having children.
Finally, it was noted that thanks to the new treatments available, the average life expectancy of patients with Cystic Fibrosis has started to increase significantly. The increase in the average life expectancy of patients means that new conditions and other health problems have to be addressed, which are found in the healthy normal population and have not been present in CF patients until now.
Reference was also made to the adverse effects that have been recorded from taking these drugs, such as the increased occurrence of rash and the risk of increasing liver enzymes over the years, and the case of a patient who experienced first-degree heart block after taking tezacaftor-ivacaftor and azithromycin for 8 months was mentioned. Discontinuation of azithromycin in this patient corrected the problem, and frequent monitoring of the electrocardiogram of patients on this treatment was suggested, so that this complication could be diagnosed and treated early.
The importance of inhaled drugs and how their administration is modified after starting treatment with CFTR modifiers was also extensively discussed. In particular, it was mentioned that the possibility of reducing the administration of inhaled drugs is in the clinical research stage with regard to Pulmozyme and hypertonic solution. The decision to discontinue them or not depends on the patient’s condition (e.g. FEV1 indication) even for patients in good condition.
However, it has been reported that the use or not of inhaled antibiotics for patients receiving CFTR modifiers is under investigation, and such a study will be delayed due to the potential risk of flare-ups and coronavirus infection.
In parallel, the European HIT-CF Europe project to provide access to new innovative drugs for CF patients with rare mutations is still ongoing. Under this project, almost 600 samples of biological material have already been taken from patients to be tested in research, giving a positive signal that a cure will soon be available for all Cystic Fibrosis patients.